Project Group 2: Acinetobacter baumannii – Biology of a Nosocomial Pathogen
- Head:
- Gottfried Wilharm
Subject
 A colony of Acinetobacter baumannii spreading on a semi-solid medium surface
Source: RKI
A colony of Acinetobacter baumannii spreading on a semi-solid medium surface
Source: RKI
Acinetobacter baumannii and related species such as A. pittii and A. nosocomialis are Gram-negative bacteria that appear as opportunistic pathogens in hospitals where they constitute a serious threat especially to immuno-compromised patients. They can cause ventilator-associated pneumonia, infections of wounds, soft-tissue and the urinary tract as well as sepsis and meningitis. In many countries they increasingly appear as multidrug resistant pathogens, restricting therapeutic options more and more. Accordingly, the World Health Organization (WHO) classified the need for research and development into novel drugs against carbapenem-resistant Acinetobacter baumannii as particularly urgent. Since our comprehension of these pathogens is still very limited, we intend to contribute to the understanding of their biology by addressing the following topics:
- Molecular characterisation of the factors determining virulence and persistence
- Elucidating mechanisms of horizontal gene transfer and adaptive resistance development
- Characterisation of metabolic pathways of the pathogen
- Identification and characterisation of novel potential drug targets
- Novel drugs against multidrug resistant Acinetobacter baumannii
- Identification of natural reservoirs of the pathogen
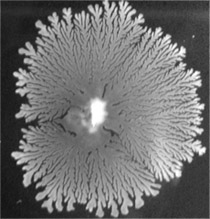
 Fungal colony (centre) with antibiotic activity against carbapenem-resistant Acinetobacter baumannii
Source: RKI
Fungal colony (centre) with antibiotic activity against carbapenem-resistant Acinetobacter baumannii
Source: RKI
Group members
MTA Evelyn Skiebe
B. Sc. Ahmad Elatris
B. Sc. Monika Saini
Open positions
Vacancies you will find advertised by the Robert Koch-Institute. Inquiries regarding practical training, bachelor, master or diploma theses can be addressed to the project leader directly.
Publications
Publications on PubMed