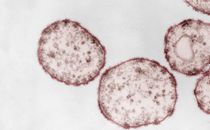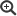
Figure legend: Mechanistic model of nucleation-dependent protein aggregation thought to underly the self-assembly and propagation of pathological Prion-Protein (PrP)-, alpha-Synuclein (αSyn)- or other proteinaceous seeds in prion-, Parkinson´s- and further neurodegenerative protein aggregation diseases, respectively. Under certain conditions, (usually monomeric) protein conformers can spontaneously, or driven by genetic factors, assemble into β-sheet-rich aggregates that constitute self-replicative protein particles, or seeds. Such initial seed formation, referred to as primary nucleation, is controlled by a high kinetic barrier. Primary nucleation is exemplarily depicted for hamster PrP, schematically showing the aggregation of PrP monomers (1) into a scrapie-associated PrP seed (2). Once proteinaceous seeds have been endogenously formed, or exogenously entered the organism, they can swiftly recruit and attach further monomers of their constitutive proteins. In this process of elongation, new aggregate mass is generated by the attachment of monomeric species to the ends of the seeding-active particles. Additionally, secondary nucleation may occur by the formation of new nucleation sites on the particle surface. When protein particles with primary or secondary nucleation sites fragment into smaller aggregates, progeny seeds enter the replication cycle and further propagate the pathological protein state. The figure is reproduced, with modifications, from Beekes M. 2021, Viruses 13:1394 (please consult this reference for all credits to image components). Source: RKI